Ll Red Drs Report Again and It Is Cervical Ascites
This commodity has been cited by other articles in ScienceCentral.
Abstract
Ascites following radical hysterectomy with retroperitoneal lymphadenectomy for invasive cervical cancer has been reported previously. Most of these reports described chylous ascites. The chylous ascitic fluid is milky; farther, chylous ascites leads to nutritional issues. Authors present the example of a patient who developed serous ascites following radical hysterectomy with bilateral pelvic lymphadenectomy. The amount of ascites was approximately xviii,000 ml over 52 days. The patient had no nutritional problems or complications. Although the etiology could not be determined, Authors surmise that the ascites may have been due to massive drainage from injured lymphatic channels below the cisterna chyli. Authors could non establish any literatures which described massive serous ascites following surgery in gynecologic malignancy and reports this example with review of literatures.
INTRODUCTION
Ascites following radical hysterectomy for invasive cervical cancer has been reported previously. Most of these reports described chylous ascites. The chylous ascitic fluid is milky; further, chylous ascites leads to nutritional problems. We present the instance of a patient who developed serous ascites following radical hysterectomy with approximately 18,000 ml of ascitic fluid over 52 days. The patient had no nutritional issues or complications. Although the etiology could not be determined, we surmise that the ascites may have been due to massive drainage from injured lymphatic channels below the cisterna chyli.
In 1994, Geisler et al. reported 2 cases of chylous ascites following retroperitoneal lymphadenectomy for gynecological malignancy.1 Postoperative intraabdominal fluid collections are occasionally encountered. Detection of ascites necessitates several evaluations, the first of which involves assessing the nature of ascites. When the color of the fluid is milky, chylous ascites is easily diagnosed. When the intraabdominal fluid is found to be serous in nature. Urine leakage acquired by an intraoperative urinary injury should be considered commencement along with other causes such as liver affliction, heart disease, postoperative intraabdominal infection, and lymph leakage from intraoperatively injured lymphatic channels. Thus far, massive intraabdominal chylous ascites and urine leakage from intraoperative urinary injuries accept been reported following extensive and radical procedures for gynecological malignancies. To our noesis, this is a rare case that presented massive serous ascites with over eighteen,000 ml that developed afterward radical hysterectomy with bilateral pelvic lymphadenectomy for locally avant-garde cervical cancer.
CASE REPORT
A 46-twelvemonth-one-time married woman consulted the Department of Obstetrics and Gynecology at Kyungpook National University Hospital in December 2004 with complaints of intermittent vaginal bleeding and foul-smelling vaginal discharge for ane year. She was diagnosed with locally invasive cervical cancer on the footing of the results of a pelvic examination, cytological smear microscopy, cystoscopy, urinalysis, colonoscopy, and magnetic resonance imaging (MRI). Prior to radical hysterectomy, she received two cycles of neoadjuvant chemotherapy with paclitaxel and carboplatin over half dozen weeks.
A pelvic examination preceding neoadjuvant chemotherapy revealed a frail and ulcerated cervical mass more than 4 cm in size involving the anterior and posterior cervical lips. In that location were no abnormal findings in the parametrial and adnexal areas.
Preoperative laboratory studies revealed the following values: Hb, 12.nine g/dl; white claret cell count, 6.99×10iii/µL with 63.3% blast cells; platelet count, 2.66×103/µL; squamous cell carcinoma antigen (S.C.C), 0.nine ng/ml; CA 125, 25 U/ml. The results of other investigations, including urinalysis, liver function tests, renal part tests, electrolytes, et al., revealed no abnormalities.
The results of electrocardiography, chest radiography, and cystoscopic test were normal. Colonoscopic examination was non possible because of big amounts of flatus and feces present within the large intestine. T-2 weighted MRI showed a 4.3 ×4. four cm hyperintensive lesion in the uterine cervix with no definitive destruction of the stromal ring (Fig. one).
Afterward 2 cycles of neoadjuvant chemotherapy, she underwent radical hysterectomy (blazon Three) with bilateral salpingoophorectomy and bilateral pelvic lymphadenectomy likewise as incidental appendectomy. In that location was no intraoperative traumas to the surrounding structures or events that could have caused massive ascites postoperatively.
Microscopic findings revealed a well-differentiated adenocarcinoma with 5-mm deep stromal invasion. Peritoneal washing cytology showed no malignant cells. Further, malignant cells were absent in all the 24 harvested pelvic lymph nodes. After radical hysterectomy, there was evidence of hematuria on the first postoperative 24-hour interval. Following irrigation of the float, no hematuria was observed. On the first postoperative day, Hb was observed to be 9.6 g/dl; this was lower than the preoperative Hb, 12.9 g/dl. The postoperative form was uneventful except for the episode of hematuria. On the postoperative days i-five, the amount of fluid obtained from the drain inserted into the pelvic lymph node dissection site was 35, 177, 257, 225, and 240 ml, respectively, on the right side, whereas on the left side, it was xxx, 35, 103, 75, and 110 ml, respectively. The fluid was yellow or straw-colored and serous in nature. On the sixth postoperative day, we removed the bleed on the left side. Transabdominal ultrasonography revealed vi ml of fluid in the posterior cul-de-sac and fluid collections measuring 18 mmthree and 21 mmthree in the right and left halves of the lower pelvic cavity, respectively. On the seventh postoperative day, ultrasonography that was performed to rule out hydronephrosis and other urinary injuries revealed no abnormal findings. Afterwards removal of the drain on the left side on the sixth postoperative day, the corporeality of fluid drainage rapidly increased upto 900 ml per day by the seventh postoperative day. We removed the drain on the correct side on the eighth postoperative day in lodge to suppress the production of ascites, following which intestinal distension developed. Computed tomography for the evaluation of intestinal distension revealed a large intraabdominal fluid drove merely no other abnormalities and no contrast leakage from the urinary tract (Fig. two). A sus scrofa-tail-shaped drain was inserted into the intraabdominal crenel for the drainage of the ascitic fluid in lodge to relieve the distension. We evaluated the nature of the fluid and the etiology of ascites. Cytology and laboratory investigations of the ascitic fluid revealed the following values: white claret jail cell count, 1,900/mm3 with 5% polymorphonucleated leukocytes and 95% lymphocytes; CA 125, 287.v U/ml; triglycerides, 6 mg/dl; adenosine deaminase, v.four IU/L; glucose, 146 mg/dl; proteins, 3.1 thousand/dl; lactate dehydrogenase (LDH), 155 U/L. Many leukocytes were observed on wet smear examination, only no organisms were observed on Gram staining, acid-fast bacilli (AFB) staining, or on culture. The differences in the BUN and creatinine levels between ascitic fluid and urine demonstrated that ascites was not due to a leakage from the urinary organization (BUN, 1.4 mmol/dl; creatinine, 0.6 mg/dl in ascites, BUN, 57.viii mmol/dl; creatinine 36.6 mg/dl in urine). No malignant or atypical cells were observed. ltrasonography revealed no aberrant findings in the liver. Breast radiography revealed no abnormalities in the lungs and the cardiac silhouette.
Intravenous pyelography for evaluation of urine leakage demonstrated no abnormal findings. From the 11th postoperative 24-hour interval, when the hog-tail-shaped drain was inserted, to the 31th postoperative mean solar day, the corporeality of serous fluid drained was observed to be 200-1,000 ml per day; thereafter, information technology decreased steadily. On the 52th postoperative day, the pig-tail-shaped drain was removed, and the patient was discharged (Fig. 3). No lymphoceles or intraabdominal fluid collections were observed during follow-upwardly.
DISCUSSION
Intraabdominal and retroperitoneal fluid collections following gynecological operations have been frequently reported. These may be detected several days or months after the functioning only are generally detected inside one week postoperatively.2 The most frequent cause of postoperative ascites is urine leakage from intraoperative injuries to the urinary tract. In this patient, we examined the possibility of a urinary injury by laboratory investigations of the ascitic fluid (the difference in the BUN and creatinine levels betwixt urine and ascitic fluid), intravenous pyelography, and computed tomography; all the same, nosotros were unable to obtain evidence of such an injury. Therefore, the possibility of lymphatic leakage following retroperitoneal lymph node dissection was considered.
Postoperative chylous ascites is produced by delayed lymphatic leakage from unhealed lymphatic channels. After injury, lymphatic channels are adhered to and compressed by the surrounding tissues, thus preventing lymphatic leakage transiently. However, failure of these channels to heal causes delayed leakage from them.2 So far, there are no definitive criteria for diagnosing chylous ascites.3 Jahsman's criteria, which are routinely used, are as follows: a milky advent, separation into ii layers on standing, odorless fluid, alkaline nature, specific gravity >4, bacteriostatic properties, total protein content <iii%, presence of fat globules that stain with the Sudan Cherry stain, fatty content of 0.4% to four%, and total solids >4%.four-7 Currently, triglyceride values have gained importance in the diagnosis of chylous ascites. These should typically exist two-8 times of the corresponding plasma values. Certain authors apply arbitrary values of ascitic fluid triglycerides to diagnose chylous ascites.eight Staats has stated that a triglyceride level >110 mg/dl is highly suggestive of chylous effusions.9 In this instance, the ascitic fluid was straw-colored or yellowish in appearance and serous in nature. The ascitic fluid triglyceride content was vi mg/dl (normal serum level, 0.4-4 mg/dl) and that of protein was 3.1 g/dl. The possibility of this fluid beingness that of chylous ascites was very low. We ruled out tuberculous ascites on the basis of the negative results of AFB stain, the absence of adenosine deaminase in the ascitic fluid, and a careful review of the patient's by history.
Imaging studies, including computed tomography, liver ultrasonography, and chest radiography, did non reveal whatever abnormal findings except for intraabdominal fluid collection. Ascites was not attributable to cardiac or hepatic causes. The incidence of chylous ascites in patients who received radiotherapy has been observed to be 3% in gynecologic malignancy. ten Nurettin et al reviewed 27 cases of chylous ascites. Of these 27 cases, 21 of them were reported to occur subsequently radiations therapy with or without previous or paraaortic lymph node autopsy.11 The about likely crusade appears to have been radiation damage to the intestine and intestinal lymphatics.12 This patient had no history of radiotherapy but had received neoadjuvant chemotherapy. In that location are no reports regarding ascites acquired by neoadjuvant chemotherapy.13,fourteen
Authors could not determine the etiology of the massive serous ascites in this patient since the laboratory values did not match the diagnostic criteria for chylous ascites; moreover, urinalysis and imaging studies were also inconclusive.
During the surgery, lymph nodes were dissected bilaterally from the common iliac nodes to the pelvic nodes but non above the level of the former. This implies that at that place was a minimal possibility of an injury to the cisterna chyli that drains the chyle from the intestinal and mesenteric lymphatics and is located anterior to the body of the L2 vertebra between the inferior vena cava and the descending aorta. In the direction of the ascites authors were deliberate on the time of drain removal. James Heaf reported boilerplate peritoneal ultrafiltration rate from 0.75 L/24-hour interval to 0.55 Fifty/day in continous ambulatory peritoneal dialysis patients.15 In the text book of surgery normally the peritoneal cavity containes less than 100 ml of sterile serous fluid. Microvilli on the apical surface of the peritoneal mesothelium promote the absorption of fluid from the peritoneal cavity into the lymphactics and the portal and systemic circulation.16 Authors could not found the reports describing when to remove and how to manage drain. On the 8th postoperative day both drains were removed in expectation of control with peritoneal transport system. Regardless of excessive ascites product or peritoneal transport failure, the ascites was not controlled. Previous studies reported that drain following retroperitoneal lymphadenectomy could not reduced postoperative lymphocysts and morbidities.17,18 The insertion and direction of drain after retroperitoneal lymphadenectomy need further report.
After excluding urinary injury and other causes authors planed to treat the patient as in chylous ascites. Many treatments have exist reported for care of chylous ascites including surgery, octreotide, somatostatin, low fatty diet with medium-concatenation triglyceride supplementation and full parenteral nutrition (TPN).xix,20 But the patients did non present any nutritional bug. The patient was treated with fluid therapy and intermittent albumin replacement without TPN and other medical treatments.
In conclusion, the possibility of massive lymphatic leakage from injured lymphatic channels tin can be suspected in this example based on the serous nature of ascitic fluid, good nutritional status of the patient, no by history of radiotherapy, and no definitive crusade of massive ascites. Authors recommend evaluation of lymphatics using lymphagiography in the example like this hereafter.
Figures and Tables
Fig. 1
Axial T2 weighted MR paradigm shows nearly iv.three×4.4 cm hypertensive bespeak on cervix.
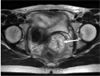
Fig. 2
Ascites. Computed tomography shows abundant ascites in abdominal cavity on 11th postoperative 24-hour interval.
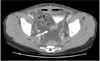
Fig. 3
Ascitic fluid drainage after radical hysterectomy with both pelvic lymphadnectomy and serum albumin level.

References
1. Geisler JP, Foster RS, Sutton GP. Chyloperitoneum post-obit treatment for avant-garde gynecologic malignancies. Obstet Gynecol. 1994. 83:883–885.
2. Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: A collective review. Surgery. 2000. 128:761–778.
![]()
iii. Hibbeln JF, Wehmueller Physician, Wilbur Air conditioning. Chylous ascites: CT and ultrasound appearance. Abdom Imaging. 1995. twenty:138–140.
![]()
4. Leibovitch I, Mor Y, Golomb J, Ramon J. The diagnosis and management of postoperative chylous ascites. J Urol. 2002. 167:449–457.
![]()
5. Baniel J, Foster RS, Rowland RG, Bihrle R, Donohue JP. Direction of chylous ascites later on retroperitoneal lymph node autopsy for testicular cancer. J Urol. 1993. 150:1422–1424.
![]()
half dozen. Ablan CJ, Littooy FN, Freeark RJ. Postoperative chylous ascites: Diagnosis and treatment. A series report and literature review. Curvation Surg. 1990. 125:270–273.
7. Jansen TT, Debruyne FM, Delaere KP, de Vries JD. Chylous ascites after retroperitoneal lymph node autopsy. Urology. 1984. 23:565–567.
![]()
8. Ward PC. Interpretation of ascitic fluid data. Postgrad Med. 1982. 71:171–173. 176–178.
![]()
9. Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein contour of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980. 55:700–704.
x. Lentz SS, Schary MF, Wilson TO. Chylous ascites after whole-belly irradiation for gynecologic malignancy. Int J Radiat Oncol Biol Phys. 1990. 19:435–438.
![]()
11. Nurettin B, Aylin PC, Gokhan T, Nejat O, K. Faruk K. Chylous ascites post-obit paraaortic lymphadenectomy: A case report. Gynecol Oncol. 2004. 93:711–714.
12. Susan LS, Michael North, John RL. Chylous ascites: A sequel of pelvic radiation therapy. Gynecol Oncol. 1985. 66:832–835.
13. Janetschek Grand, Hobisch A, Hittmair A, Höltl L, Peschel R, Bartsch G. Laparoscopic retroperitoneal lymphadenectomy after chemotherapy for stage IIB nonseminomatous testicular carcinoma. J Urol. 1999. 161:477–481.
![]()
14. Baniel J, Foster RS, Rowland RG, Bihrle R, Donohue JP. Complications of postal service-chemotherapy retroperitoneal lymph node dissection. J Urol. 1995. 153:976–980.
fifteen. James H. Pathogenic furnishings of a high peritoneal send rate. Semin Dial. 2000. 13:188–193.
16. Richard HT, Benjamin DLL, John CM. Townsend Courtney One thousand., Daniel Beauchamp R., Mark Evers B., Mattox Kenneth, editors. Intestinal wall, navel, peritoneum, mesenteries, omentum and retroperitoneum. Sabiston Textbook of Surgery. 2004. 17th ed. Philadelphia, Pennsylvania: Elsevier Saunders;1181–1182.
17. Franchi M, Trimbos JB, Zanaboni F, v d Velden J, Reed North, Coens C, et al. Randomized trial of drains versus no drains following radical hysterectomy and pelvic lymph node autopsy: A European Organization for Research and Handling of Cancer-Gynecological Cancer Grouping(EORTC-GCG) study in 234 patients. Eur J Cancer. 2007. 43:1265–1268.
18. Srisomboon J, Phongnarisorn C, Suprasert P, Cheewakriangkrai C, Siriaree South, Charoenkwan K. A prospective randomized written report comparing retroperitoneal drainage with no drainage and no peritonealization post-obit radical hysterectomy and pelvic lymphadenectomy for invasive cervical cancer. J Obstet Gyneacol Res. 2002. 28:149–153.
19. Al-Ghamdi MY, Bedi A, Reddy SB, Tanton RT, Peltekian KM. Chylous ascites secondary to pancreatitis: management of an uncommon entity using parenteral nutrition and octreotide. Dig Dis Sci. 2007. 52:2261–2266.
![]()
xx. Tom PM, Shahab A, Jeffrey MF. Chylous ascites post-obit treatment for gynecologic malignancies. Gynecol Oncol. 2002. 86:370–374.
Source: https://synapse.koreamed.org/articles/1123464
0 Response to "Ll Red Drs Report Again and It Is Cervical Ascites"
Post a Comment